Tests for infectious diseases
Lateral flow immunoassay - immunoassay based on the principle of thin layer chromatography and included reaction between antigen and relevant antibody in the biological materials (urine, whole blood, serum or plasma, saliva, feces, etc.).
This type of assay is carried out using test strips, panels or test cassettes, which ensure the speed of testing. LFIA is rapid test.
Being an effective diagnostic tool, rapid tests allow you to visually determine and evaluate the content of antigens, antibodies, hormones and other diagnostically important substances in the human body within a few minutes. Rapid tests are characterized by a high degree of sensitivity and accuracy detecting more than 100 types of diseases, including such common diseases as tuberculosis, syphilis, gonorrhea, chlamydia infection, various types of viral hepatitis, etc., as well as the entire range of narcotic drugs used with a high reliability of determination. An important advantage of this type of tests is their use in vitro diagnostics which does not require the direct presence of the examined patient.
ИХА-анти-ВГС-ФАКТОР
The kit is intended for one-step rapid qualitative determination of the presence of antibodies to hepatitis C virus (HCV) in vitro in human serum, plasma or whole blood by lateral flow immunoassay.
ИХА-HbsAg-ФАКТОР
The kit is intended for in vitro visual rapid one-step qualitative determination of the presence of hepatitis B virus surface antigen (HBsAg) in human serum, plasma or whole blood method.
ИХА-анти-НВc-ФАКТОР
The kit is intended for in vitro rapid one-step qualitative determination of antibodies to hepatitis B core antigen (HBc) in human whole blood, serum of plasma by lateral flow immunoassay
ИХА-анти-ТП-ФАКТОР
The kit is intended for in vitro one-step rapid qualitative determination of the presence of antibodies to Treponema pallidum (syphilis) in human serum, plasma or whole blood by lateral flow immunoassay
ИХА-ХЛАМИДИЯ-ФАКТОР
The kit is intended for in vitro one-step rapid qualitative determination of Chlamydia trachomatis in biological samples (cervical smears in women and urethral smears in men) by lateral flow immunoassay
ИХА-ГОН-ФАКТОР
The kit is intended for in vitro visual rapid one-step qualitative determination of infectious agent causing gonorrhea (Neisseria gonorrhoeae) in biological samples (epithelial cell scraping from cervical mucous membrane in women or urethra in women and urethra in men) by lateral flow immunoassay.
ИХА-анти-НР-ФАКТОР
The kit is intended for in vitro rapid one-step qualitative determination of antibodies to Helicobacter pylori (HP) in human whole blood, serum and plasma by lateral flow immunoassay.
ИХА-ЦитоМВ-ФАКТОР
Test is intended for in vitro rapid one-step qualitative determination of IgM cytomegalovirus antibodies (CytoMV) in serum, plasma or whole blood by lateral flow immunoassay.
ИХА-ТБ-ФАКТОР
The kit is intended for in vitro one-step rapid qualitative determination of antibodies to Mycobacterium tuberculosis in serum, plasma or whole blood by lateral flow immunoassay
ИХА-ВИЧ-1/2-ФАКТОР
The kit is intended for in vitro visual rapid one-step qualitative determination of antibodies to human immunodeficiency virus type 1 and/or 2 (HIV 1/2) in human serum, plasma or whole blood by rapid test.
ИХА-ВИЧ-1-АГ-ФАКТОР
The kit is intended for in vitro visual rapid one-step qualitative determination of HIV-1 p 24 antigen in human serum, plasma or whole blood by lateral flow immunoassay.
Enzyme immunoassay (EIA) - method for detection antigens and antibodies based on the determination of antigen-antibody complex due to introduction of enzyme label in one of reaction components with its further detection using relevant substrate changing its color. The basis for any variant of EIA is the determination of enzyme reaction products in study of testing samples compared to negative and positive controls.
To determine antigens and antibodies, a solid-phase (heterogeneous) variant of the enzyme immunoassay is used The use of a solid phase allowed simplification of the process of separating the reaction components by immobilizing one of the components on the solid phase and removing the substances not participating in the reaction.
Enzyme immunoassay has the following advantages over other methods for detecting antigens and antibodies:
- high sensitivity, allowing to detect concentrations up to 0.05 ng/ml;
- the ability to use the minimum volume of the test material;
- storage stability of all ingredients required for EIA;
- the simplicity of the reaction;
- the presence of both instrumental (qualitative and quantitative) and visual accounting;
- the ability to automate all stages of the reaction;
- relatively low cost of diagnostic kits.
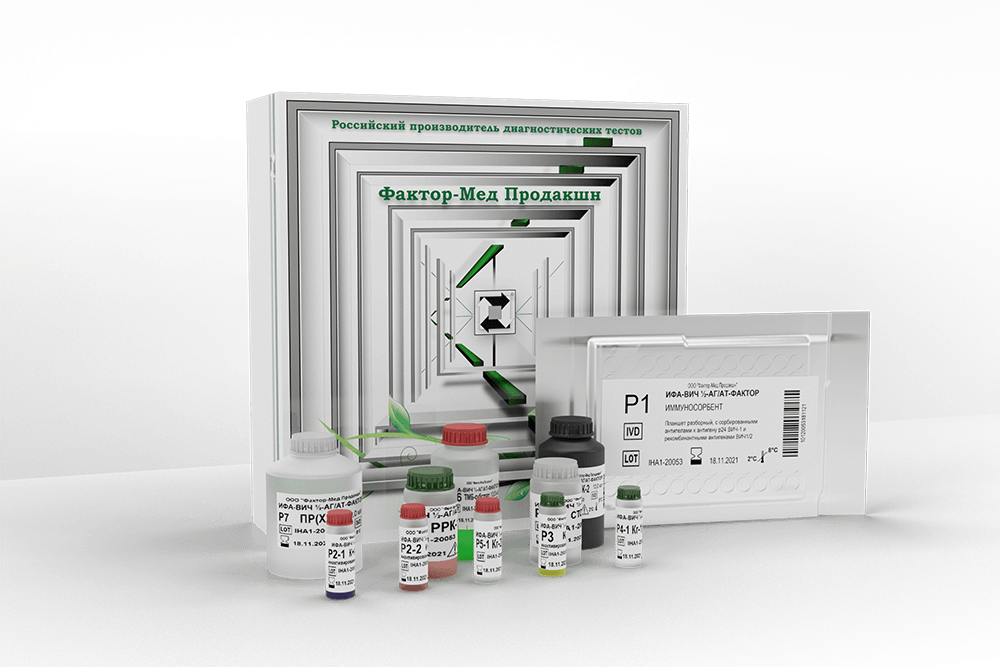
ИФА-ВИЧ1/2-АГ/АТ-ФАКТОР
The kit is intended for screening and confirmation tests of human serum and plasma for the presence of HIV 1 p24 antigen and total antibodies (IgG, IgM, IgA) to HIV-1 and HIV-2 by the enzyme immunoassay.

ИФА-ВИЧ-1-АГ-ФАКТОР
The kit is intended for testing human serum or plasma for the presence of HIV-1 p24 antigen by enzyme immunoassay.
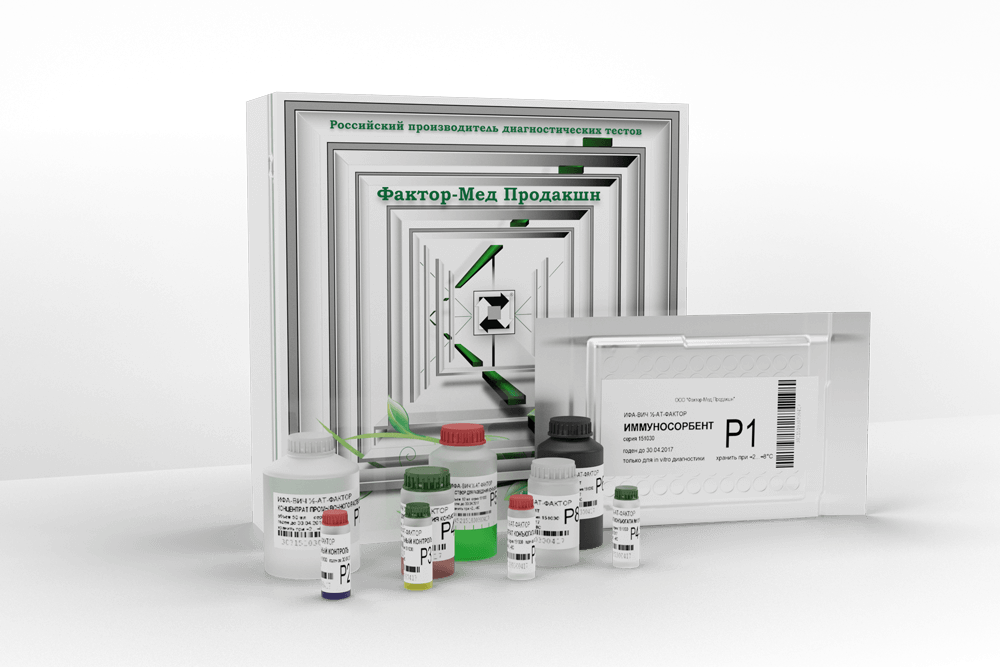
ИФА-ВИЧ1/2-АТ-ФАКТОР
The kit is intended for the determination of specific antibodies of IgG, IgM, IgA classes to human immunodeficiency virus types 1 and 2 in human serum or plasma by enzyme immunoassay.

ИФА-анти-ТП-ФАКТОР
The kit is intended for testing human serum or blood plasma for the presence of IgG and IgM antibodies to Treponema pallidum (syphilis) by enzyme immunoassay.
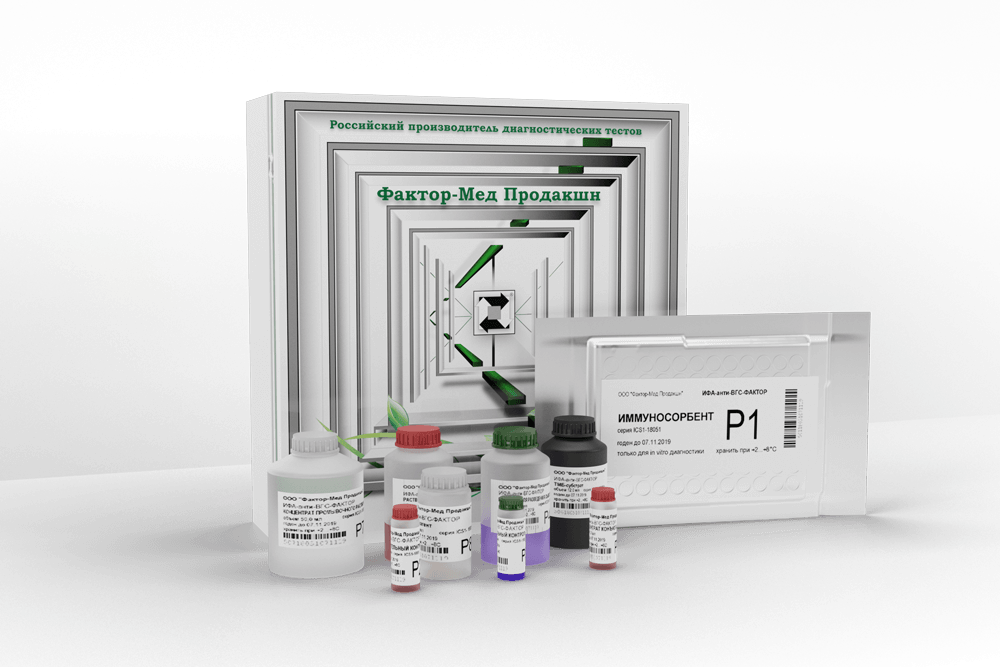
ИФА-анти-ВГС-ФАКТОР
The kit is designed to detect IgG antibodies to hepatitis C virus proteins in human serum or plasma by enzyme immunoassay both in early stages of seroconversion and in chronic course of the disease.
